|
Case Series
Clustering of Familial pancreatic adenocarcinoma in a family from Sudan
1 Consultant Physician of Internal Medicine and Medical Oncology, Ambulatory Medicine, Hamad, Medical Corporation, Doha-State of Qatar
2 Pre-college Volunteer Student at London Central Secondary School, Applicant for Medical School at Weill Cornell-Qatar (WCM-Q)
Address correspondence to:
M. S. Ahmed
Consultant Physician of Internal Medicine and Medical Oncology/Ambulatory Medicine, Hamad Medical Corporation, P.O. Box - 3050,
Doha-State of Qatar
Message to Corresponding Author
Article ID: 100008C01MA2018
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Ahmed MS, Sadiq YM. Clustering of Familial pancreatic adenocarcinoma in a family from Sudan. Edorium J Cancer 2018;4:100008C01MA2018.ABSTRACT
Introduction: Pancreatic cancer (PC) is an aggressive and rapidly progressive malignancy with a 5-year survival rate of approximately 5%. Of all pancreatic cancers, 80% are adenocarcinomas of the ductal epithelium and only 2% of tumors of the exocrine pancreas are benign. On first diagnosis, half of patients found to have distant disease and half of the rest are locally advanced. Many genetic factors, hereditary syndromes, and lack of screening programs contributed to the behavior of PC.
Case Series: We report familial pancreatic cancer in four members of the same family, two brothers diagnosed to have pancreatic cancer at their fifth decade of age. Previously their sister was diagnosed with adenocarcinoma of the cecum at fifth decade, and their mother died of colorectal cancer at old age. The presentation of the three siblings were similar in term of tumor markers, as CA 19–9 (tumor marker of pancreatic cancer) was within normal limits at diagnosis in all three patients, but CEA was slightly more than normal and their lower endoscopies showed no colorectal polyps but diverticulosis. The histological diagnosis of both brothers with PC was poorly-differentiated adenocarcinoma of the pancreas. This is a first report from Qatar describing this rare clinical entity.
Conclusion: Familial pancreatic cancer would behave similar to pancreatic cancer in clinical context but it may be easy to screen for bearing in mind its behavior to affect many individuals in the same family; especially if we can find the underlying precursors to explain its pathophysiology. The aim of this article is to increase physicians’ awareness of this clinical entity.
Keywords: Familial pancreatic cancer, Pancreatic cancer, Screening
INTRODUCTION
Pancreatic cancer (PC) is still a highly lethal disease with a 5-year survival rate of approximately 5 % [1]. Of all pancreatic cancers, 80% are adenocarcinomas of the ductal epithelium. Only 2% of tumors of the exocrine pancreas are benign. As in literature PC is affecting higher age group >60 years [2]. Pancreatic cancer is notoriously difficult to diagnose in its early stages. At the time of diagnosis, 52% of all patients have distant disease and 26% have regional spread [3]. Risk factors include tobacco smoking, obesity, high alcohol consumption, history of pancreatitis and diabetes, family history of pancreatic cancer, and possibly selected dietary factors [4]. Only 5-10% are hereditary in nature. In the absence of predisposing conditions, such as familial pancreatic cancer and chronic pancreatitis, pancreatic cancer is unusual in persons younger than 45 years. After age 50 years, the frequency of pancreatic cancer increases linearly. Familial predisposition characterizes up to 10% of the patients with pancreatic cancer [5].
Relatives of patients with pancreatic cancer and persons with certain inherited syndromes such as familial pancreatic cancer (FPC) are at increased risk for developing pancreatic cancer in their lifetime. In this article, we report FPC in a Sudanese family, to increase our physician awareness of this clinical entity.
CASE SERIES
Case 1
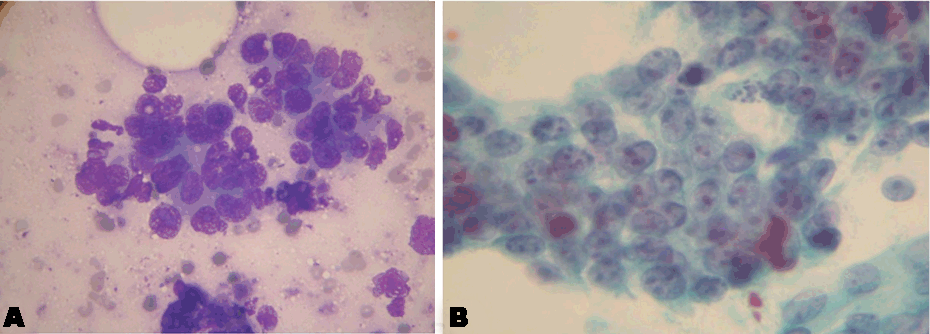
This is a 54-year-old male patient, presented to National Center for Cancer Care and Research (NCCCR) in State of Qatar, on March 2010, with persistent constant colicky epigastric pain of five months duration, occasionally radiating to the right shoulder and to the back. The patient was a retired military colonel, married and had children. His medical history included cigarette smoking 2 packets/ day, but no alcohol abuse, drug abuse, chronic pancreatitis, diabetes mellitus, or occupational exposure to toxic chemicals. His physical examination was remarkable for tender epigastric mass. Because of the nature of his unremitting pain, evaluation for possible metastatic disease was initiated. CAT scan revealed a tumor (4.8x5.8x5 cm) arising from the root of mesentery involving a group of moderately enlarged lymph nodes, and the uncinate process of the pancreas, the tumor posterior surface touching the third part of duodenum, encircling and compressing the superior mesenteric vein but no visceral nor distant metastases were found in the liver or lungs, and even bone scan showed clear skeleton of metastases. Tumor markers showed normal CA19-9 and high CEA of 6.3 (normal up to 3 mcg/ml). Subsequently, CAT guided fine needle aspiration showed pancreatic adenocarcinoma (Figure 1A and 1B).
The general surgeon found the tumor locally advanced and inoperable intra-operatively, so laparatomy incision closed after peritoneal wash. Three weeks later, the patient was started on palliative chemotherapy in form of IV Gemcitabine 1000 mg/m2, Day 1 and IV Oxaliplatin 100 mg/m2, Day 2, repeated every two weeks but the tumor did not respond and the mass remained unchanged, moreover; the CEA increased up to 12.8 mcg/ml, so after completion of 4 cycles the patient was shifted onto new chemo protocol consist of IV Gemcitabine (above dose) on Day 1 & 8, combined with Capecitabine orally, 1000 mg/m2 BID for 14 days.
The cycle repeated every three weeks, after four cycles the tumor slightly regressed in size and the CEA decreased to 8 mcg/ml. Unfortunately, the patient relapsed with increasing CEA reaching 20 mcg/ml post 6th cycle of same chemo protocol, for which Erlotinib was added in a dose of 100 mg/day to the backbone of Gemcitabine instead of the previous chemo protocol and we managed to keep the tumor mass size stable and the CEA back to 12 mcg/ml. We are intending to continue with same regimen as far as the patient will remain tolerable with the disease stable, otherwise ,shift onto other regimen if disease progresses or relapsed or patient becomes intolerable.
Case 2
This second patient, is an older brother of the above patient, 57-years-old, he presented to NCCCR on May 2010, with a history of five kilogram weight loss and epigastric pain over two months duration. The patient worked in business, married and had children. He has no history of smoking, alcohol abuse, drug abuse, chronic pancreatitis, diabetes mellitus, or occupational exposure to toxic chemicals. On physical examination, he appeared as fully alert pale middle aged man with vital signs as : BP 137/ 81, Pulse 92/ minute regular, RR 23/minute, abdominal exam showed tender left upper tender quadrant mass, firm in consistency and fixed to the underlying structures, while head, neck, chest and heart were normal. He suffered from severe anemia (Hemoglobin 6.4 gm/dl, iron deficiency anemia) and weight loss, his lower endoscopy indicated and confirmed the presence of ulcerated friable mass after crossing the splenic flexure proximally, along with diverticulae in the colonic wall here and there, and large external hemorrhoids, one of them is thrombosed, subsequently a biopsy from the ulcerated mass showed malignant cells consistent with adenocarcinoma, CEA and CA19-9 were within normal, CT scan for staging showed a pancreatic mass infilterating into the wall of colonic splenic flexure but no distant metastases in the liver and the lungs, also the bone scan ruled out bony metastases.
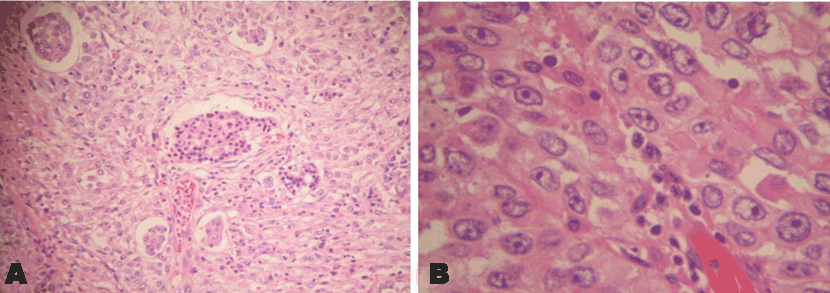
The patient underwent whipple’s surgery involving en-bloc resection of the body and tail of pancreas, along with the spleen, splenic flexure of colon, greater curvature of stomach, macroscopically the tumor size was 10X8X7.5 cm, and microscopically, it was poorly differentiated invasive pancreatic adenocarcinoma (Figure 2A and 2B), invading the whole thickness of colonic wall up to the mucosa and into the stomach as well, no lymph nodes were involved out of 10 resected, with free resection margins.
Because the tumor capsule was ruptured and slightly fragmented at the peripheries, it was prudent to have the patient started on postoperative chemotherapy with adjuvant intent, 20 doses of IV gemcitabine 1000mg/m2 were planned, first seven doses given on weekly basis then followed by cycles consisted of Day 1, 8 and 15, repeated every four weeks, the patient completed 14 doses with a very good tolerance and decided to go back to his home country, Sudan, to continue treatment balance of six more cycles there. Unfortunately, the patient condition deteriorated in Sudan due to cancer progression and intra-abdominal metastases before completing the adjuvant chemo and he expired in his hometown hospital.
Case 3
A 55-years-old postmenopausal, widowed female, sister of the above two patients, had suffered from vague abdominal colicky pain for six months associated with fatigue, dyspnoea on exertion and weight loss of eight kilogram over last two months. Her past medical history remarkable for appendicectomy during early adulthood and caesarean section at 35 years of age for dysfunctional uterine bleeding. She had no history of smoking, alcohol abuse, drug abuse, chronic pancreatitis, diabetes mellitus, or occupational exposure to toxic chemicals. She is housewife and a mother of six children.
Her physical examination revealed pallor and had a relatively mobile, non-tender mass of six cm in right iliac fossa. Laboratory investigations showed hypochromic microcytic anemia of 7.5 gm/dl.
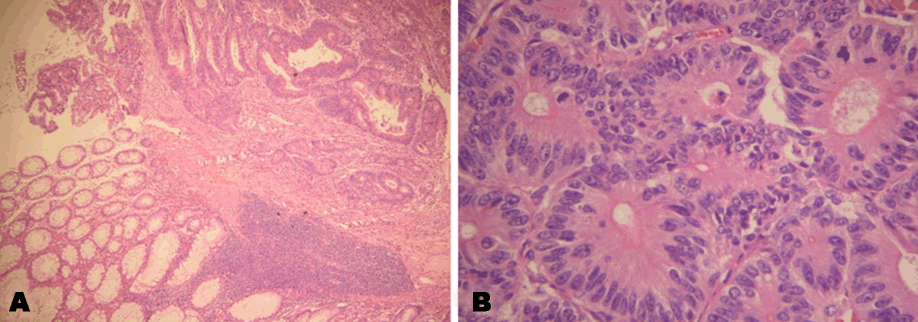
On mid-July 2008, in Al-Khartoum-Sudan, CT imaging revealed cecal tumor which was confirmed by colonoscopy to be a polypoidal, fungating growth along with discrete divertculae in the wall of the colon, histopathological diagnosis of the growth was moderately differentiated colonic adenocarcinoma (Figure 3A & 3B). No other distant metastases were found through imaging scans. She presented to State of Qatar for treatment where Right hemicolectomy was done on 30 july 2008, staged as stage IIa, T3 N0 M0, no metastases were found in 31 lymph nodes resected. Thereafter she was offered adjuvant chemotherapy but she declined and went back home.
Case 4
The mother of the above three siblings, at the age of 7-years, was diagnosed to have metastatic gastrointestinal cancer in Sudan based on endoscopy and radiology but unfortunately no records were available to explore them, and she died because of the disease progression within short time of diagnosis.
DISCUSSION
Familial Pancreatic Cancer (FPC) is defined by families with at least a pair of first-degree relatives who have been diagnosed with PC and do not fulfill the criteria of other inherited tumor syndromes [6]. Increasingly younger age at presentation is a characteristic feature of many forms of familial cancer. It was found that half of FPC probands are males, and the mean age at diagnosis of affected members is younger (64 to 65 years) than expected compared to sporadic pancreatic cancer patients. The original evidence supporting the hypothesis that some proportion of pancreatic cancer might have a genetic basis was drawn from observations of familial clustering and hereditary syndromes that featured increased risk of pancreatic cancer [7]. Multiple hereditary syndromes such as Peutz-Jeghers syndrome, hereditary pancreatitis, hereditary breast and or ovarian cancers with positive BRCA1 & 2 gene mutations, hereditary nonpolyposis colorectal cancer (HNPCC), and familial adenomatous polyposis, also have increased risks of PC, but they do not fit the very specific definition of FPC [8].
Familial pancreatic cancer would follow an autosomal dominant inheritance with heterogeneous phenotype but there is no known gene defect to be blamed for this entity so far although germline mutations in BRCA2, PALB2 and ATM are found in some FPC families [9]. We believe that our case series would support this global concept and will strongly substantiate the importance of launching an effective means of screening against FPC.
In view of this data which indicated that familial factors play a contributory role to this disease, experts think that early detection and prevention might offer the best hopes for reducing the mortality from pancreatic cancer. Pancreatic cancer usually diagnosed in advanced stage, when it became hard to treat or to cure; in other words, it is difficult to diagnose at an early stage. PC often presents with variable presentation that makes it even more difficult to distinguish from more common disorders until late when it became locally advanced or metastatic. For instance, it is recommended to consider pancreatic cancer as a differential diagnosis in diabetic patients who presented with unusual manifestations such as unexplained weight loss and or vague abdominal symptoms.
At International meetings experts generally agreed to appropriately screen for pancreatic cancer in individuals at higher risk through multidisciplinary approach. Yet all attempts, until present time, were unsuccessful to establish recognized guidelines for specific tumor markers, gene mutations or a suitable imaging technique to screen for or to diagnose highly suspicious pancreatic tumors, what is called precursor lesions [10]. Currently suggested screening techniques are based on findings from endoscopic ultrasonography and MRI, and data has demonstrated that with the help of these modalities precursor lesions for pancreatic cancer would possibly be identified.
We would like to draw our clinicians’ attention, primary care or family physicians in particular, for FPC & to have a lower threshold to recommend early screening on healthy members in families with history of FPC. Same recommendations have to be applied on high-risk patients with a history of smoking, recurrent pancreatitis, or one of the syndromes mentioned above, or other well recognized genetic predisposing factors. If such suspicions are confirmed the above candidates will be saved from an early death by prophylactic pancreatectomy or image-guided radiosurgery.
CONCLUSION
Retrospective to this case series report and many other similar case reports of FPC it appeared that this type of aggressive malignancy has the tendency to inflict families and or to associate other gastrointestinal cancers in the same family. This fact must be considered as a potential to screen healthy members in the same family. International research is warranted; first to explain the pathophysiology of FPC and second to explore an appropriate cost effective imaging technique to screen for FPC in the near future.
REFERENCES
1.
Wada K, Takaori K, Traverso LW, et al. Clinical importance of familial pancreatic cancer registry in Japan: A report from kick-off meeting at international symposium on pancreas cancer 2012. Hepatobiliary Pancreat Sci 2013 Aug;20(6):557–66. [CrossRef]
[Pubmed]

2.
Medscape. Pancreatic cancer. [Available at: https://emedicine.medscape.com/article/280605-overview#a2]

3.
American Cancer Society. Cancer Facts & Figures 2017. [Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf]

4.
Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: An update. Dig Dis 2010;28(4–5):645–56. [CrossRef]
[Pubmed]

5.
Mastoraki A, Chatzimavridou-Grigoriadou V, Chatzipetrou V, et al. Familial pancreatic cancer: Challenging diagnostic approach and therapeutic management. J Gastrointest Cancer 2014 Sep;45(3):256–61. [CrossRef]
[Pubmed]

6.
Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: An overview. Nat Rev Gastroenterol Hepatol 2009 Dec;6(12):699–708. [CrossRef]
[Pubmed]

7.
Matsubayashi H, Takaori K, Morizane C, et al. Familial pancreatic cancer: Concept, management and issues. World J Gastroenterol 2017 Feb 14;23(6):935–48. [CrossRef]
[Pubmed]

8.
Petersen GM. Familial pancreatic cancer. Semin Oncol 2016 Oct;43(5):548–53. [CrossRef]
[Pubmed]

9.
Bartsch DK, Gress TM, Langer P. Familial pancreatic cancer: Current knowledge. Nat Rev Gastroenterol Hepatol 2012 Aug;9(8):445–53 [CrossRef]
[Pubmed]

10.
Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013 Mar;62(3):339–47. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
M. S. Ahmed - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Y. M. Sadiq - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this case series.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2018 M. S. Ahmed et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.