|
Research Article
Primary medullary adenocarcinoma of the colon: Literature review and case series
1 MBBS, PGDipAnat, Faculty of Medicine University of Tasmania, Tasmania, Australia; General Surgical Department, Royal Hobart Hospital, Tasmania, Australia
2 MBBS, Department of Surgery, Royal Hobart Hospital, Tasmania, Australia
3 MBBS, Anatomical Pathology, Royal Hobart Hospital, Tasmania, Australia
4 MBBS, Faculty of Medicine University of Tasmania, Tasmania, Australia; General Surgical Department, Royal Hobart Hospital, Tasmania, Australia
5 MBBS, Department of Surgery, St George Hospital, New South Wales, Australia
6 MBBS, FRACS, Royal Hobart Hospital, Tasmania, Australia
7 MBBS, FRACS, Faculty of Medicine University of Tasmania, Tasmania, Australia; General Surgical Department, Royal Hobart Hospital, Tasmania, Australia
Address correspondence to:
Hein Maung
48 Liverpool St, Hobart, Tasmania 7000,
Australia
Message to Corresponding Author
Article ID: 100012C01HM2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Maung H, Gregory O, Hoog TD, Hutchinson M, Soh PB, Marino M, Evans T, Yeoh A, Turner RC. Primary medullary adenocarcinoma of the colon: Literature review and case series. Edorium J Cancer 2024;8(1):1–10.ABSTRACT
Aims: Medullary carcinoma of the colon is a rare subtype of adenocarcinoma, first described in 1999. Clinically known to have a favorably prognosis in comparison to poorly differentiated cancers, it is invariably associated with mismatch gene repair. This is an observational study of Hobart’s patient population with medullary cancer, and compares data with the current literature.
Methods: We performed a search of the pathological database at our institution for medullary adenocarcinomas between the years of 2016 and 2023 and reviewed their clinical information to collect all relevant data including patient history, hospital admissions, surgery and clinic visits. We then performed a literature search using PubMed for search terms medullary cancer/carcinoma of the colon/colorectum.
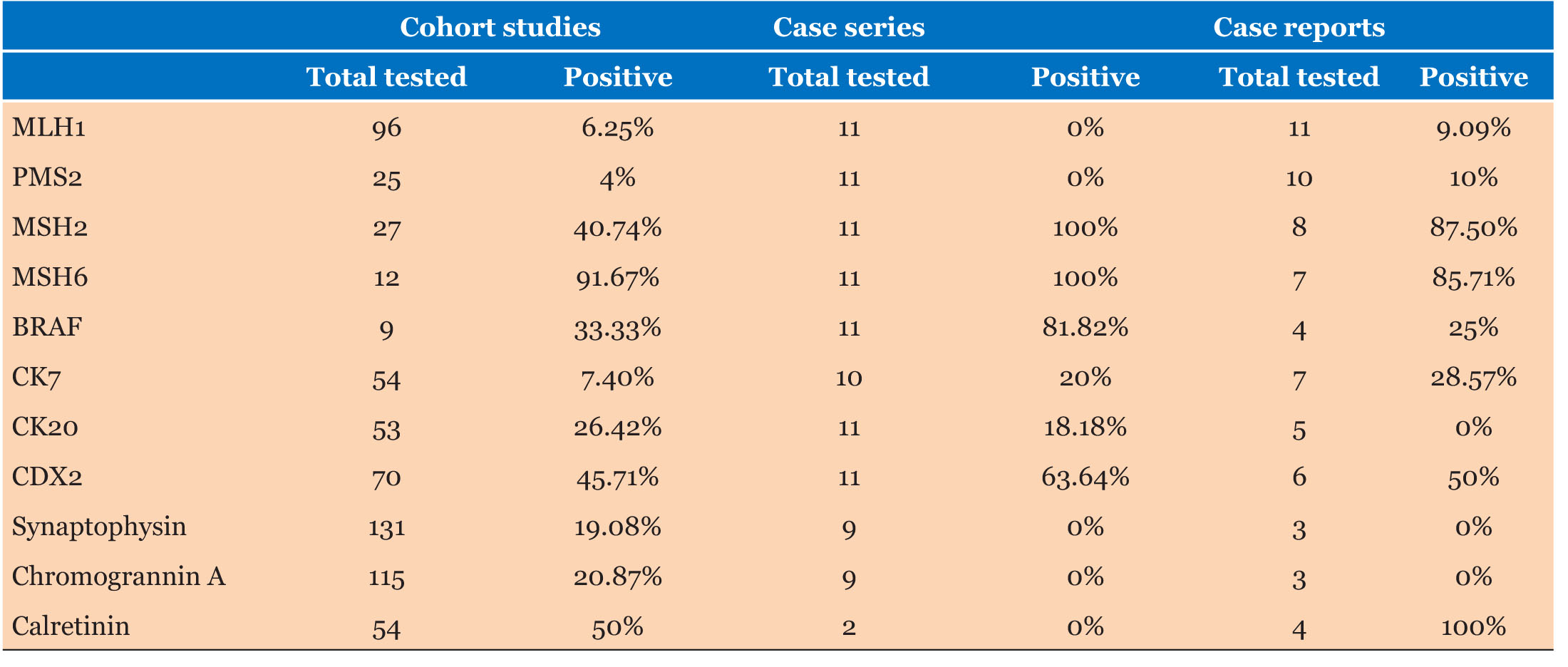
Results: Eleven patients were found in our database, 34 papers in the literature (19 retrospective cohort studies and 13 case reports). 81.8% (vs. 73.22% in cohort studies) were females. 8/11 patients had lymphovascular invasion (LVI) with 2/11 patients had perineural involvement (PNI). The immunohistochemistry (IHC) results showed that in all (11/11) patients’ tumors, there was a loss of MLH1 and PMS2 proteins, while MSH2 and MSH6 proteins were present. Cohort studies demonstrated 302/1897 (15.92%) patients had perineural invasion (PNI) with 1133/2151 (52.67%) demonstrating LVI. MLH1 testing was available for 192 patients, with 93.75% having loss of MLH1.
Conclusion: Our cohort of medullary cancer patients was similar to that in the literature, with regard to demographic, staging, and tumor characteristics. A longer follow-up time is required for our cohort to produce comparable survival outcomes.
Keywords: Colon cancer, Colorectal, Primary medullary colon cancer
INTRODUCTION
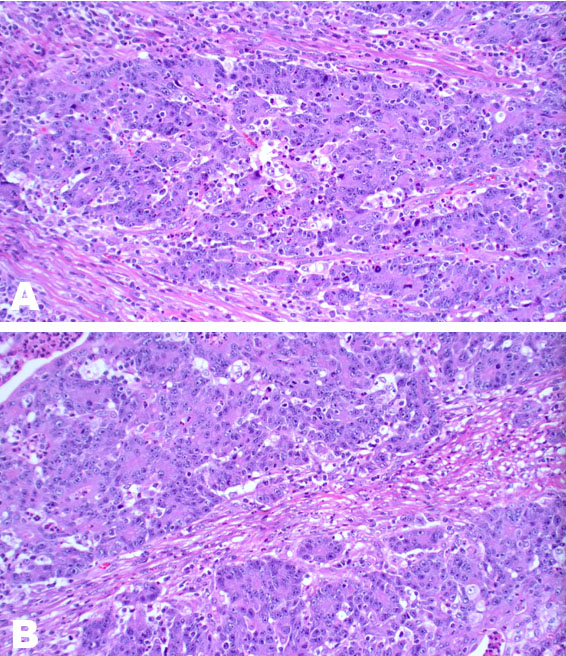
Medullary carcinoma (MCC) of the colon is a recently recognized but rare subtype of colonic adenocarcinoma, with the first published case documented in 1999 [1],[2]. It is not to be conflated with breast and thyroid cancers because the clinicopathological features are quite different [3]. It has a strong association with Lynch syndrome and sporadic causes of mismatch repair (MMR) deficiency, without the usual immunohistochemical (IHC) staining pattern or staining results, seen in colorectal adenocarcinoma [4]. Clinically this subtype is associated with a favorable prognosis when compared to other poorly differentiated/undifferentiated colorectal cancers of a similar grade and is invariably associated with mismatch gene repair deficiency [5],[6]. It has a propensity to affect older women >65 years of age more than men, has a lower risk of lymph node spread and occurs more often on the right side of the colon [4],[7]. Histologically, MCC can be misclassified as poorly differentiated adenocarcinoma not otherwise specified (NOS) [8]. It is typically characterized by sheets of malignant cells with indistinct cell boundaries, vesicular nuclei, prominent nucleoli, abundant eosinophilic cytoplasm, and prominent intratumoral lymphocytes and neutrophils. It invariably presents with microsatellite instability [9].
Given MCC is a rare subtype of colorectal cancer (CRC), and there is some literature pertaining to this subtype, we aim to present data from a local Australian perspective and compare it to the current literature.
MATERIALS AND METHODS
A search of the anatomical pathology database at our institution for medullary cancers of the colon between 2016 and 2023 was performed and patients’ digital medical record was assessed. Data were extracted on clinical history, operation notes, investigations, and outpatient notes.
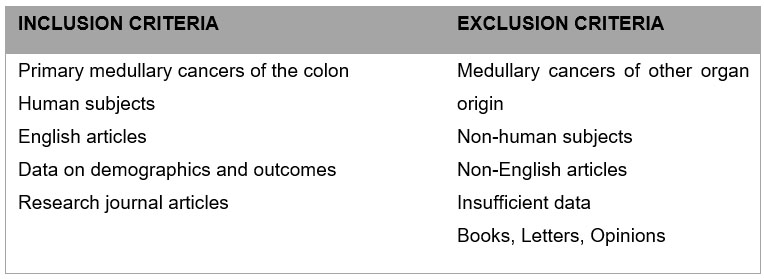
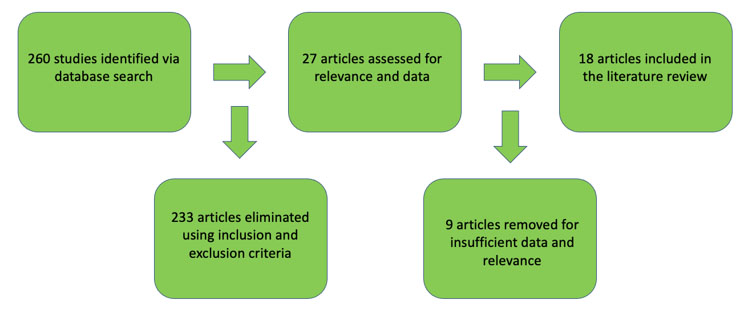
For the literature search, search terms “colon,” “rectum,” “colorectal,” “medullary carcinoma,” and “solid type poorly differentiated carcinoma” were entered to retrieve relevant articles in the PubMed and MEDLINE databases up to August 2023 using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta- Analyses) guidelines. Titles and abstracts were screened for relevance and exclusion. Original and review articles were searched utilizing the same inclusion/exclusion criteria (Figure 1). The initial search yielded 260 results (Figure 2 and Figure 3). These articles then were filtered by 2 authors, then the 18 abstracts were read, and secondary references searched where indicated. Secondary references were utilized and duplicates were eliminated for a result of 402 articles. After filtering the abstracts according to criteria, 13 articles were then read and included in the study. The combination of the initial search (18 articles), and a search of the references (13 articles) yielded a total of 31 articles—13 case reports and 18 retrospective cohort studies for our literature review. One of the papers in the references was a meta-analysis of medullary cancer of the colon from 2016—the papers that informed this study were included in our final count [5].
Approval for our case series was obtained from the Tasmanian Human Research Ethics Committee (ref. no. 29765).
RESULTS
Overall
Eleven patients at the institution had MCC in their pathology over seven years of our database search. We found 18 retrospective cohort studies with a total of 3158 patients, as well as 13 case reports involving MCC of the colon, one of which documented 2 patients.
Age and sex
Nine (81.8%) of the 11 patients in our local database were females with a ratio of 3:1 compared to males; ages of patients ranged from 56 to 93, with a mean age of 75.3 (Table 1). Average age in case reports was 68.5 years. Cohort studies’ results had an average age of 70.5 years; 73.2% were females (out of 3144 patients due to clinical data availability).
Comorbidities and presentation
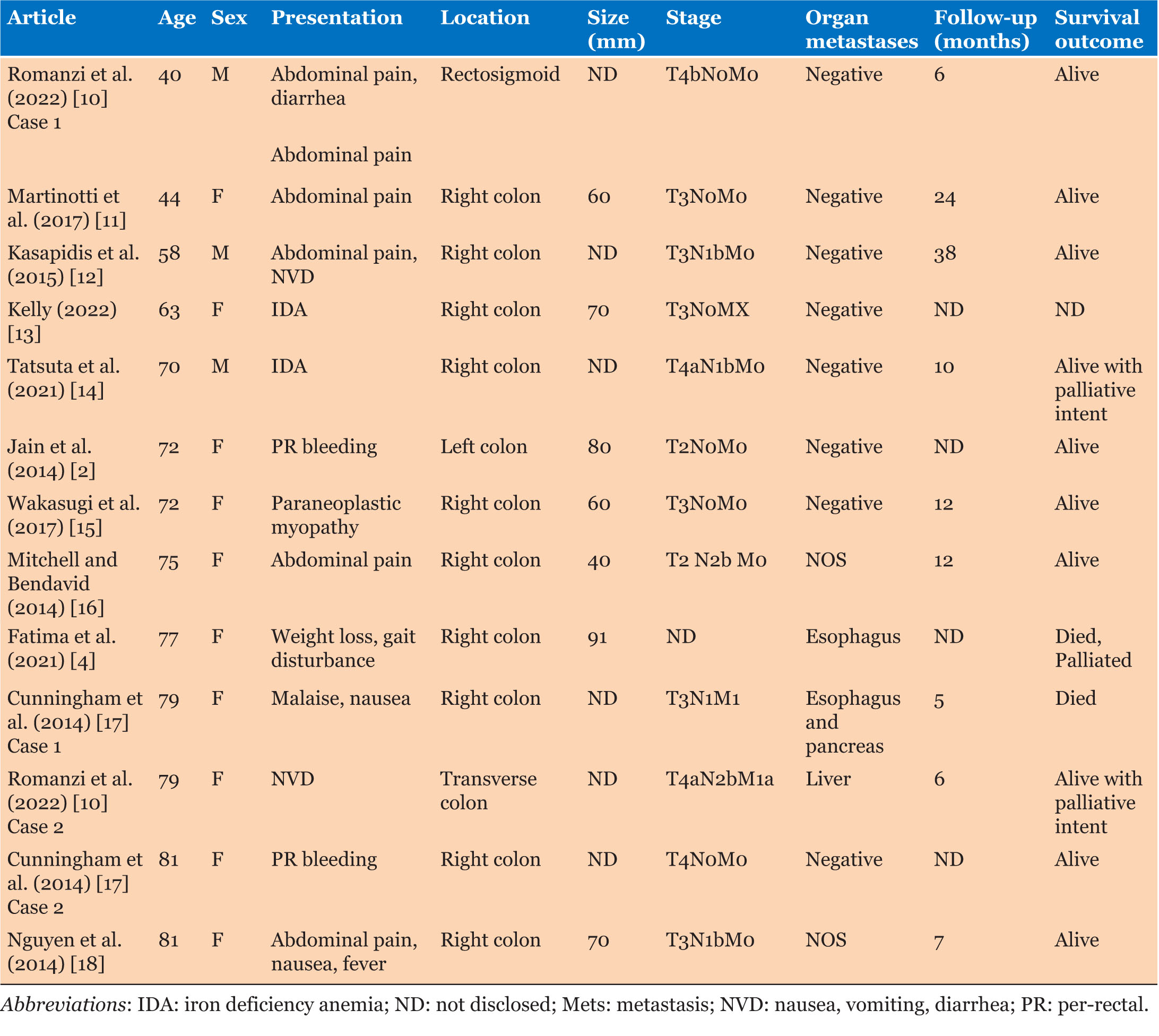
Case series (Table 1)
Four (36.4%) of 11 patients were ex-smokers or obese, with 2 (18.2%) patients having previous primary malignancies—breast and prostate/colon cancer, respectively. The patient with prostate cancer also had a previous history of a colonic adenocarcinoma which was treated with surgery alone. Four (36.4%) patients presented with iron deficiency anemia (IDA) and a positive fecal occult blood test, while 2 (18.2%) patients presented via the Australian National Bowel Screening program (NBSP).
Case reports
Average age of patients was 68.5 years, with MCC predominantly affecting females 10 of 13 (76.9%). Three of 14 (21.4%) patients had ischemic heart disease, and only one patient was an ex-smoker. Two of 11 (18.2%) patients had other primary malignancies—multiple myeloma and melanoma of the chest. Six of 14 (42.9%) patients presented with abdominal pain, and 4 (28.6%) patients had either iron deficiency anemia or per-rectal (PR) bleeding (Table 2).
Cohort studies
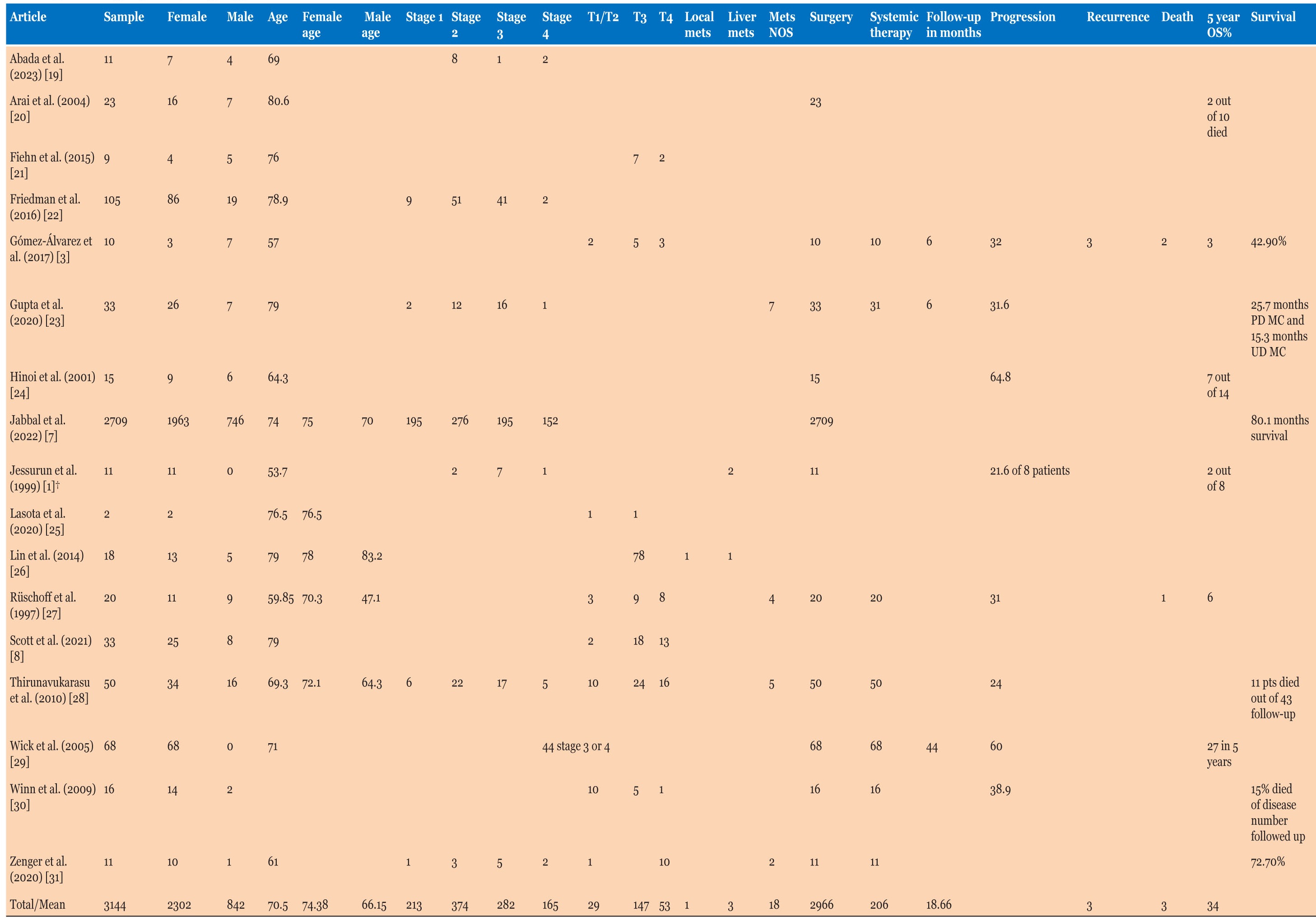
Cohort studies had a sample of 3144 patients, with 2302 (73.2%) being females, with an average age of 74.4 years (Table 3), different from males who presented at a younger age of 66.2 years. Three studies mentioned patient factors like smoking, obesity, diabetes, and other cancers. Four of 21 (19.1%) patients were overweight/obese, and the same number were smokers. One study had a body mass index (BMI) average of 28.1 in a cohort of 11. Only one study mentioned previous primary malignancies in 10 of 50 (20%) cases. None of the cohort studies had data on the presentation symptoms of patients.
Carcinoembryonic antigen (CEA), Tumor size, and Location
Case series
Only 6 patients had CEA tested prior to surgery, with an average of 4.05 µg/L (normal 0–2.5 µg/L) and a range of 1.1–8.8 µg/L. Tumor size was 57.3 mm on an average in maximal dimension, with a range of 40–95 mm, and 10 of 11 (90.9%) were within the right colon, with one (9.1%) in the transverse colon.
Case reports
Only 5 cases had CEA documented without exact levels, it was elevated in 20% of those patients. Tumor size was documented in 8 cases with an average of 67 mm, and 10 of 13 (76.9%) involved the right colon.
Cohort studies
455 of 1571 (29%) patients had raised CEA levels and one study had an average CEA of 2.8 µg/L in a sample of 11 patients. Tumor size was 70 mm with an average range of 63.8–96 mm in 6 studies, and 2700 of 3113 (86.7%) were discovered in the right colon.
Pathological staging and TNM staging
Case series
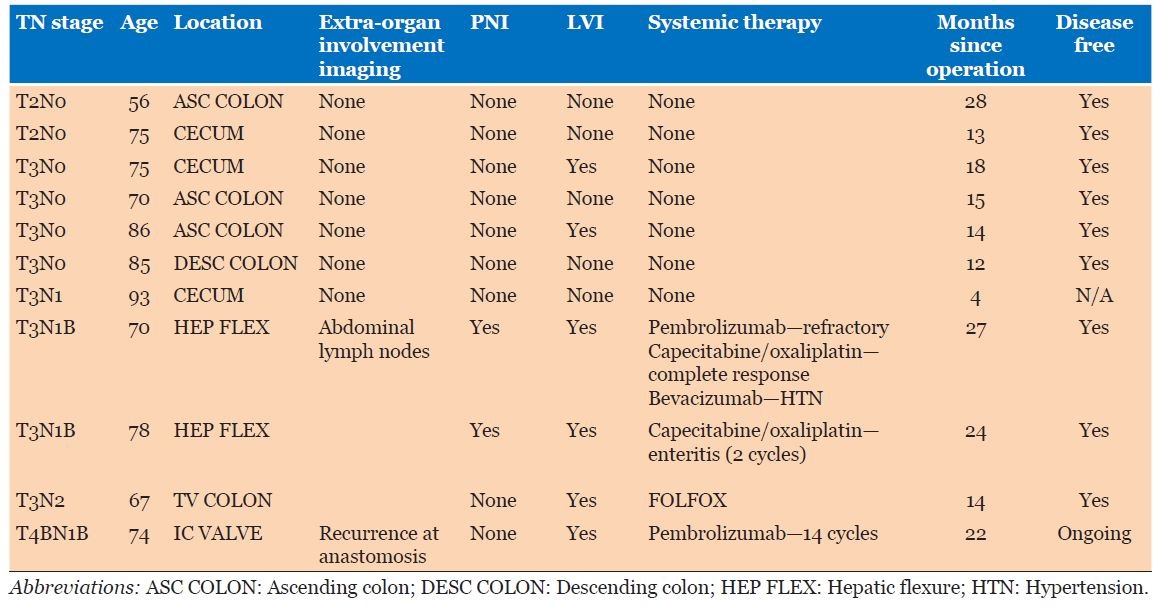
Eight of 11 (72.7%) patients had tumors T3 or higher, with 5 of 11 (45.5%) involving lymph nodes. There were no distant metastases; however, one patient had early recurrence at the anastomotic site, prompting a change in their systemic therapy regimen. Five (45.5%) patients had stage 3 disease or higher according to American Joint Committee on Cancer (AJCC) staging.
Case reports
Nine of 13 (69.2%) patients had T3 or higher tumors, with 7 of 13 (53.9%) patients with stage 3 or higher MCC. 5 of 13 (38.5%) patients had extra-organ metastases with spread to the liver or paraesophageal lymph nodes and pancreas (Table 2).
Cohort studies
Patients with stage 3 or higher disease were 447 of 1034 (43.2%), with 200 of 229 (87.3%) patients with T3 or higher disease (where study has explicitly mentioned T stage). Nearly 39% of patients had lymph node metastases (1087 of 2812) and 22 of 143 patients had extra organ metastases.
Treatment, follow-up, and survival
Case series
All patients proceeded to a right hemicolectomy, and 5 (45.5%) had node positive disease. Of these 5 patients, 4 were treated with systemic therapy, but 1 treated with surgery alone due to age. All patients are currently alive as of submission, and follow-up was an average of 17 months (range 4–28 months). One patient is still having ongoing systemic therapy of Pembrolizumab due to early recurrence (<6 months post-operatively) at the anastomotic site, with paraaortic lymph node involvement, and the highest grade of tumor T4bN1b—this is no longer detectable on latest imaging. None of the case series patients have died.
Case reports
Four of 9 (44.4%) patients received systemic therapy post-operatively, 2 patients were treated palliatively due to age and comorbidities but were alive at time of writing the reports. Death was reported in 2 of 12 (16.7%) with one patient dying due to complications of systemic therapy and one due to post-operative complications. Follow-up had a mean of 13.3 (range 6–38) with an average range of 5–38 months in 10 cases.
Cohort studies
Of the 5 studies that mentioned systemic therapy, 151 of 155 (97.4%) patients had surgery and 65 of 155 (41.9%) patients had systemic therapy. Eleven studies mentioned survival in a multitude of ways with 53% survival in 7 studies, and 2 studies mentioning a 5-year overall survival of 42.9% and 72.7%. Two studies measured survival in months with one study differentiating poorly differentiated MCC with a 25.7 month mean survival months vs. 15.3 months in undifferentiated MCC, and another study defining survival at 80.1 months on average. Follow-up data in 8 studies averaged to 21.4 months ranging from 8–44 months (Table 3).
Tumor characteristics, BRAF and KRAS mutation
Case series
Eight of 11 patients had lymphovascular invasion and 2 of 11 demonstrated perineural invasion (Table 1). Immunohistochemistry (IHC) demonstrated loss of MLH1 and PMS2 expression in the tumors of all 11 patients with presence of MSH2 and MSH6. BRAF V600e mutations were absent on staining in 2 of 11 (18.8%) of patients and stains CK7, CK20, and CDX2 were positive in 20%, 18.2%, and 63.6% respectively.
Case reports
There was sparse data on tumor characteristics with 1 of 3 samples having PNI, and LVI 2 of 3 (Table 3). Similarly, for KRAS and BRAF mutations, only 5 patients were tested for either. Two of 4 patients were BRAF positive, and 1 of 3 patients was KRAS wild type. MLH1 was negative in the tumors of 10 of 11 patients (90.1%).
Cohort studies
Out of 1897 patients, 302 (15.9%) had PNI with 1133 of 2151 (52.7%) demonstrating LVI. MLH1 testing was available for 192 patients, with 93.8% having loss of MLH1. Stains CK7, CK20, and CDX2 were 7.4%, 26.4%, and 45.7% positive, respectively. Four studies mentioned KRAS mutation with 238 of 313 (76%) (Table 4).
DISCUSSION
Given MCC’s reported incidence of 0.03% [10] of all colorectal cancers and its unique clinicopathological, immunohistochemical, and prognostic profile, there is limited data currently available about this rare subtype. This study demonstrates some interesting findings regarding MCC.
The mean and median age in our case series was 75 years, compared to the average of 70 years in the pooled cohort studies. The occurrence of MCC among female patients was also higher at a ratio of 3:1, compared to 2:1 in the literature [28], which included one study with an overrepresentation of males at 70% [3].
Six of 11 patients presented with iron deficiency anemia or a positive fecal occult blood test investigated by the Australian National Blood Supply Contingency Plan (NBSCP) or their general practitioner (GP), reinforcing the importance of age-based screening with reduction of mortality by 23% [32].
American Joint Committee on Cancer Stage 3 disease was higher in our group with 45.5% vs. 38.7% in the literature. Contrary to much of the literature, one study demonstrated a poorer prognosis in MCC patients with stage 3 disease vs. other non-MCC cancers with a survival of 15.3 months vs. 47.2 months p=0.001 [13]. Location of the tumor was in the right colon with 100% of our patients undergoing a right hemicolectomy. Right colonic involvement was similar in the literature review with 86.7% involvement of the colon. Tumor size was smaller in our cohort at 57 mm, with 70 mm average in the cohort studies, and generally MCC is known to present with larger sizes in comparison to other poorly differentiated or undifferentiated adenocarcinomas [7].
Survival in our case series was 100% vs. 16.6% mortality in the pooled case reports and 53% in the observational cohort studies. This is likely biased by our short follow-up time of 17 months average (range 4–28 months). One patient had an early recurrence of disease at the anastomotic site despite having a clear margin resection, with TNM score of T4bN1bM0.
Pathologically, MCCs can be differentiated from other undifferentiated carcinomas by strong calretinin staining and loss of MLH1 and CDX2 staining [10], often CDX2 is positive in colorectal adenocarcinoma [33]. Our case series identified 2 specimens where calretinin was negative, with lack of expression of CK20, CK7, and chromogranin A with weak or focal CDX2 staining. However, in these cases, SATB2, which has been demonstrated to be positive in 95% of colorectal cancers [34], demonstrated positive staining while MLH1 and PMS2 were absent. Morphologically both tumors favored a diagnosis of MCC.
There are limitations with this study. Firstly, with regard to our case series, the cohort was too small a number to make any definitive conclusions about MCC. Due to the small population size, the follow-up period had a large range, and none of the patients reached 5-year survival mark at the time of writing the study. Patient demographic data on BMI and smoking history were surprisingly sparse. Pathological staining was variable; however, our institution routinely investigated MMR proteins and BRAF.
Pertaining to the literature review, the search could have been improved by performing our search on a third database, and given MCC relatively new classification, there may be other terms outside of uncommon search terms such as solid-type poorly differentiated adenocarcinoma—this term however was included.
CONCLUSION
Medullary carcinomas tend to affect females more than males, and generally occur in the right side of the colon. Given the rarity of this sub-type, surgeons and pathologists are encouraged to continue contributing longitudinal data to inform future management pathways.
REFERENCES
1.
Jessurun J, Romero-Guadarrama M, Manivel JC. Medullary adenocarcinoma of the colon: Clinicopathologic study of 11 cases. Hum Pathol 1999;30(7):843–8. [CrossRef]
[Pubmed]

2.
Jain S, Jain A, Onizuka N, Boukhar SA. A rare case of medullary carcinoma of the colon presenting as intussusception in an adult with rectal bleeding. Hawaii J Med Public Health 2014;73(11):348–52.
[Pubmed]

3.
Gómez-Álvarez MA, Lino-Silva LS, Salcedo- Hernández RA, et al. Medullary colonic carcinoma with microsatellite instability has lower survival compared with conventional colonic adenocarcinoma with microsatellite instability. Prz Gastroenterol 2017;12(3):208–14. [CrossRef]
[Pubmed]

4.
Fatima Z, Sharma P, Youssef B, Krishnan K. Medullary carcinoma of the colon: A histopathologic challenge. Cureus 2021;13(6):e15831. [CrossRef]
[Pubmed]

5.
Pyo JS, Sohn JH, Kang G. Medullary carcinoma in the colorectum: A systematic review and meta-analysis. Hum Pathol 2016;53:91–6. [CrossRef]
[Pubmed]

6.
Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76(2):182–8. [CrossRef]
[Pubmed]

7.
Jabbal IS, Nagarajan A, Rivera C, et al. Medullary carcinoma of the colon: A comprehensive analysis of the National Cancer Database. Surg Oncol 2022;45:101856. [CrossRef]
[Pubmed]

8.
Scott N, West NP, Cairns A, Rotimi O. Is medullary carcinoma of the colon underdiagnosed? An audit of poorly differentiated colorectal carcinomas in a large national health service teaching hospital. Histopathology 2021;78(7):963–9. [CrossRef]
[Pubmed]

9.
10.
Romanzi A, Centonze G, Sabella G, et al. Colorectal medullary carcinoma: Heterogeneous presentations of a rare clinico-pathological entity. Report of two cases. Tumori 2022;108(6):NP20–5. [CrossRef]
[Pubmed]

11.
Martinotti M, Cirillo F, Ungari M, et al. Microsatellite instability in medullary carcinoma of the colon. Rare Tumors 2017;9(1):6541. [CrossRef]
[Pubmed]

12.
Kasapidis P, Grivas E, Papamichail V, Alfaras P. Medullary carcinoma of the colon: An adenocarcinoma with better prognosis. Ann Gastroenterol 2015;28(2):289.
[Pubmed]

13.
Kelly ML. Medullary carcinoma in the colon. ANZ J Surg 2023;93(3):710–11. [CrossRef]
[Pubmed]

14.
Tatsuta K, Sakata M, Iwaizumi M, et al. Mismatch repair proteins immunohistochemical null phenotype in colon medullary carcinoma. Clin J Gastroenterol 2021;14(5):1448–52. [CrossRef]
[Pubmed]

15.
Wakasugi M, Kono H, Yasuhara Y, et al. A resected case of medullary carcinoma of the ascending colon followed by infarction of the greater omentum mimicking anastomotic leakage. Int J Surg Case Rep 2017;41:456–60. [CrossRef]
[Pubmed]

16.
Mitchell A, Bendavid Y. Medullary colon cancer presenting with total necrosis of all regional lymph node metastases: Morphologic description of a presumed immune-mediated event. Diagn Pathol 2014;9:204. [CrossRef]
[Pubmed]

17.
Cunningham J, Kantekure K, Saif MW. Medullary carcinoma of the colon: A case series and review of the literature. In Vivo 2014;28(3):311–4.
[Pubmed]

18.
Nguyen J, Coppola D, Shan Y, Zhang L. Poorly differentiated medullary carcinoma of the colon with an unusual phenotypic profile mimicking high grade large cell lymphoma – A unique case report and review of the literature. Int J Clin Exp Pathol 2014;7(2):828–34.
[Pubmed]

19.
Abada E, Jang H, Kim S, Abada O, Beydoun R. Medullary colonic carcinomas present with early-stage disease and do not express neuroendocrine markers by immunohistochemistry. Ann Gastroenterol 2023;36(3):321–6. [CrossRef]
[Pubmed]

20.
Arai T, Esaki Y, Sawabe M, Honma N, Nakamura K, Takubo K. Hypermethylation of the hMLH1 promoter with absent hMLH1 expression in medullary-type poorly differentiated colorectal adenocarcinoma in the elderly. Mod Pathol 2004;17(2):172–9. [CrossRef]
[Pubmed]

21.
Fiehn AMK, Grauslund M, Glenthøj A, Melchior LC, Vainer B, Willemoe GL. Medullary carcinoma of the colon: Can the undifferentiated be differentiated? Virchows Arch 2015;466(1):13–20. [CrossRef]
[Pubmed]

22.
Friedman K, Brodsky AS, Lu S, et al. Medullary carcinoma of the colon: A distinct morphology reveals a distinctive immunoregulatory microenvironment. Mod Pathol 2016;29(5):528–41. [CrossRef]
[Pubmed]

23.
Gupta A, Protyniak B, Dove J, et al. A comparison of treatments and outcomes for medullary versus nonmedullary colon cancer: A single institutional experience showing a worse prognosis for stage 3 disease. Surg Res Pract 2020;2020:5783729. [CrossRef]
[Pubmed]

24.
Hinoi T, Tani M, Lucas PC, et al. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am J Pathol 2001;159(6):2239–48. [CrossRef]
[Pubmed]

25.
Lasota J, Chłopek M, Wasąg B, et al. Colorectal adenocarcinomas harboring alk fusion genes: A clinicopathologic and molecular genetic study of 12 cases and review of the literature. Am J Surg Pathol 2020;44(9):1224–34. [CrossRef]
[Pubmed]

26.
Lin F, Shi J, Zhu S, et al. Cadherin-17 and SATB2 are sensitive and specific immunomarkers for medullary carcinoma of the large intestine. Arch Pathol Lab Med 2014;138(8):1015–26. [CrossRef]
[Pubmed]

27.
Rüschoff J, Dietmaier W, Lüttges J, et al. Poorly differentiated colonic adenocarcinoma, medullary type: Clinical, phenotypic, and molecular characteristics. Am J Pathol 1997;150(5):1815–25.
[Pubmed]

28.
Thirunavukarasu P, Sathaiah M, Singla S, et al. Medullary carcinoma of the large intestine: A population based analysis. Int J Oncol 2010;37(4):901–7. [CrossRef]
[Pubmed]

29.
Wick MR, Vitsky JL, Ritter JH, Swanson PE, Mills SE. Sporadic medullary carcinoma of the colon: A clinicopathologic comparison with nonhereditary poorly differentiated enteric-type adenocarcinoma and neuroendocrine colorectal carcinoma. Am J Clin Pathol 2005;123(1):56–65.
[Pubmed]

30.
Winn B, Tavares R, Fanion J, et al. Differentiating the undifferentiated: Immunohistochemical profile of medullary carcinoma of the colon with an emphasis on intestinal differentiation. Hum Pathol 2009;40(3):398–404. [CrossRef]
[Pubmed]

31.
Zenger S, Gurbuz B, Can U, Balik E, Bugra D. Clinicopathologic features and prognosis of histologic subtypes in the right-sided colon cancer. J BUON 2020;25(5):2154–9.
[Pubmed]

32.
Lew JB, St John DJB, Macrae FA, et al. Benefits, harms, and cost-effectiveness of potential age extensions to the national bowel cancer screening program in Australia. Cancer Epidemiol Biomarkers Prev 2018;27(12):1450–61. [CrossRef]
[Pubmed]

33.
Moskaluk CA, Zhang H, Powell SM, Cerilli LA, Hampton GM, Frierson HF Jr. Cdx2 protein expression in normal and malignant human tissues: An immunohistochemical survey using tissue microarrays. Mod Pathol 2003;16(9):913–9. [CrossRef]
[Pubmed]

34.
Magnusson K, de Wit M, Brennan DJ, et al. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. Am J Surg Pathol 2011;35(7):937–48. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgments
We would like to acknowledge Dr. Sunit Sarkar, Oncology Department, Royal Hobart Hospital for his contributions toward this publication.
Author ContributionsHein Maung - Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Oliver Gregory - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Thomas De Hoog - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Matthew Hutchinson - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Pith Beh Soh - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Matthew Marino - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Tobias Evans - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Adrian Yeoh - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Richard C Turner - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Hein Maung et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.