|
Research Article
Outcome of patients with glioblastoma treated with Stupp protocol, 10-year review: A single center retrospective study
1 Department of Medical Oncology, Mid-West Cancer Centre, University Hospital Limerick, Limerick, Ireland
2 Mid-West Radiation Oncology Centre, University Hospital Limerick, Limerick, Ireland
Address correspondence to:
Ruba A Hamed
Department of Medical Oncology, Mid-West Cancer Centre, University Hospital Limerick, Limerick,
Ireland
Message to Corresponding Author
Article ID: 100011C01RH2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Hamed RA, Mahgoub T, Lowry L, Elbassiouni M, Korpanty G. Outcome of patients with glioblastoma treated with Stupp protocol, 10-year review: A single center retrospective study. Edorium J Cancer 2022;7:100011C01RH2022.ABSTRACT
Aims: Glioblastoma (GBM) is the most aggressive malignant brain tumor. In 2005, Stupp et al. demonstrated an increase in median overall survival (OS) to 14.6 months using radical radiotherapy (RT) with concurrent and adjuvant temozolomide (TMZ). We aim to review the outcome and OS of patients treated with the Stupp regimen in our center.
Methods: Retrospective analysis of 64 patients diagnosed with GBM between 2008 and 2018 and treated under Stupp protocol. Data collected from electronic records and survival analysis was completed using the Kaplan–Meier method.
Results: The median age was 59 years; 70% (45) were male. 76.6% (49) underwent debulking surgery, and 23.4% had biopsy only. 78% (50) completed concurrent RT and TMZ. 65.6% (42) proceeded to have adjuvant TMZ with a median of 5.5 cycles. The median OS for the whole cohort was 13.2 months (95% CI 10–15.4). Median OS for patients who had debulking surgery was 14.5 months, versus 11.1 months for patients with biopsy (p=0.62). Patients who received adjuvant TMZ after concurrent RT-TMZ had OS of 15.6 months, compared to 9.6 months for patients who did not receive adjuvant TMZ (p=0.004).
Conclusion: The median OS survival of our cohort was slightly inferior to what was observed in the Stupp trial.
Keywords: Glioblastoma, Outcome, STUPP, Temozolomide
INTRODUCTION
Glioblastoma (GBM) is the most aggressive type of gliomas, a collection of tumors arising from glia or their precursors within the central nervous system (CNS). It is the most common primary brain and CNS malignancy, accounting for 45.2% of primary malignant brain and CNS tumors, 54% of all gliomas, and 15.6% of all primary brain tumors [1].
Glioblastoma is primarily diagnosed at an older age, the median age of diagnosis is 64 years, and the incidence is slightly higher in males than females [2],[3].
The clinical presentation can vary depending on the size, location of the tumor, and the anatomic structures of the involved brain [4],[5]. Patients usually present with symptoms of increased intracranial pressure, including headache and focal or progressive neurologic deficits. Seizure is a common presenting symptom that occurs in 25% of patients. In the advanced stage of the disease, seizures can occur in about 50% of patients [5],[6].
The prognosis of GBM is poor; tumors generally recur after standard multimodal treatments, and most patients survive only 12–18 months from diagnosis [7].
Surgery represents the initial treatment, and it is usually feasible in 60% of the patients [7]. The extent of resection represents a significant prognostic variable, and multiple studies have demonstrated the importance of aggressive surgical resection when possible, with trends toward better outcomes in those patients with a greater extent of resection [8],[9], and significant association between the greater extent of resection and more prolonged progression-free survival (PFS) and overall survival (OS) [10],[11],[12].
Postoperative radiation therapy (RT) alone was standard treatment until 2005 when the results of a randomized phase III trial by the European Organization for Research and Treatment of Cancer (EORTC) and National Cancer Institute of Canada Clinical Trials Group (NCIC) changed the standard of care for GBM. This trial confirmed adjuvant external beam RT with concomitant temozolomide (TMZ); oral alkylating chemotherapy was more effective than RT alone [13].
In the study, after surgical resection, TMZ (75 mg/m2/day×7 days/week for six weeks) was administered concomitantly with RT (total dose of 60 Gy delivered by a schedule of 2 Gy/day×5 days/week for six weeks (phase 1) and in the adjuvant setting (150–200 mg/m2/day×5 days, every 28 days for six cycles (phase 2), known as the Stupp regimen [13].
Patients who received TMZ plus RT had a median survival of 14.6 months versus 12.1 months with RT alone, and two years survival rate was 26.5% with RT plus TMZ group and 10.4% with radiotherapy alone.
The long-term results of the trial published in 2009 demonstrated that the 5-year survival rates of the patients treated with the combined therapy and those receiving RT alone were 9.8% and 1.9%, respectively [14]. A further retrospective analysis of methylation status of MGMT from tumor tissues of 206 patients showed that methylation of MGMT was a strong predictor of better outcomes from TMZ treatment, and patients whose tumor contained a methylated MGMT promoter were most likely to benefit from the addition of TMZ to RT [14].
In our study, we retrospectively investigated the outcome of patients treated with Stupp protocol in our center between 2008 and 2018.
Ethical approval was obtained from the local Research Ethics Committee.
MATERIALS AND METHODS
All patients diagnosed with GBM referred at our Cancer Center between 2008 and 2018 were identified from electronic records. Those treated with Stupp protocol had confirmed pathology, and complete records were included in the analysis. Demographics planned and delivered treatments, total cycles of adjuvant TMZ, toxicity, and outcome were recorded. We then identified the date of death for these individuals from electronic records.
The survival time was calculated from the date of diagnosis to the date of death, or the date of analysis (April 2020) for those patients still alive at the time of data collection. The primary endpoint of the analysis was overall survival. Kaplan–Meier curves were used for survival analyses, and Cox proportional Hazard Model was used for p-value calculation.
RESULTS
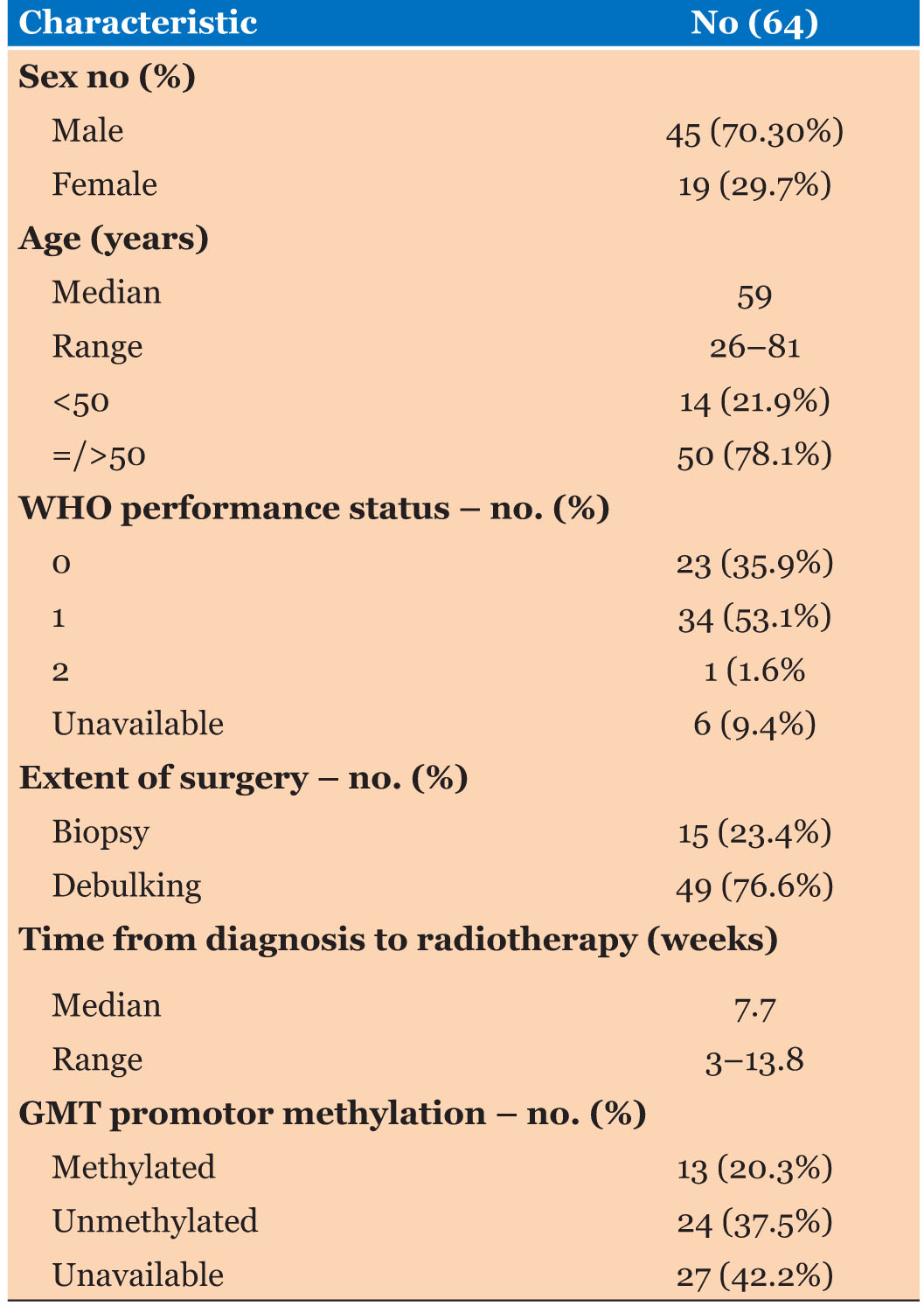
A total of 64 patients were identified who had commenced Stupp protocol between 2008 and 2018 for histologically proven GBM. The median age was 59 years (range 26–82 years), the majority of patients were male 70%, and about 78% were 50 years or older. Around 90% of the patients had a WHO performance status of 0 or 1. Most of the patients (76.6%) had undergone debulking surgery, and 23.4% had biopsy only. Thirteen patients (20.3%) had tumors exhibiting MGMT promoter methylation, 24 tumors (37.5%) were unmethylated, and MGMT methylation status was unavailable for 27 tumors (42.2%). The median time from diagnosis to start concurrent therapy was 7.7 weeks (range 3–13.8 weeks), and the median dose of RT was 60 Gy (Table 1).
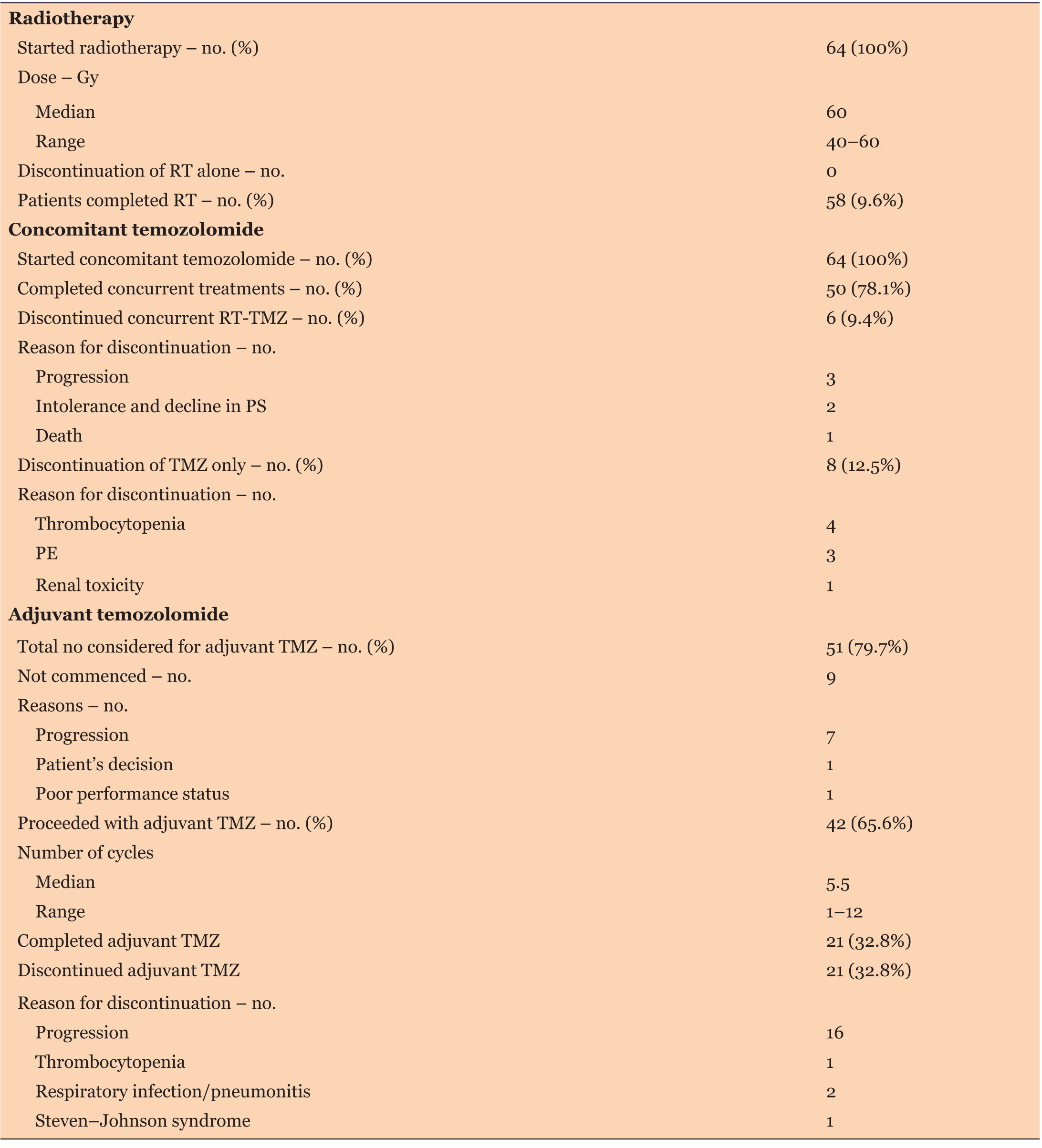
Among the 64 patients, 50 patients (78.1%) completed concurrent RT-TMZ as planned, 6 patients (9.4%) prematurely discontinued concurrent RT and TMZ. Treatment discontinuation was due to disease progression in 3 patients, intolerance in 2 patients, and death in 1 patient. Eight patients (12.5%) discontinued TMZ only and continued with RT alone; reasons for discontinuation of TMZ were thrombocytopenia (in 4 patients), pulmonary embolism (in 3 patients), and renal toxicity (in 1 patient).
Regarding phase two adjuvant TMZ, 51 patients (79.7%) were considered for adjuvant TMZ, including one of the patients who discontinued TMZ in the concurrent setting due to thrombocytopenia. Nine of these patients did not proceed due to the progression of disease (7 patients), poor performance status (1 patient), and personal decision (1 patient). Forty-two patients (65.6%) proceeded to have adjuvant TMZ and received a median of 5.5 cycles (range 1–12), only half of them completed standard six adjuvant cycles of TMZ or more with an average of 6.8 cycles (17 patients received six cycles, 1 received seven cycles, 1 received nine cycles, and 2 received 12 cycles).
Twenty-one patients (32.8%) received less than six cycles of adjuvant temozolomide. Reasons for discontinuation were disease progression in 15 patients, death in 1 patient, toxicities in 4 patients (thrombocytopenia, Steven Johnson’s syndrome, pneumonitis, and respiratory infection), and 1 patient due to persistent brain edema.
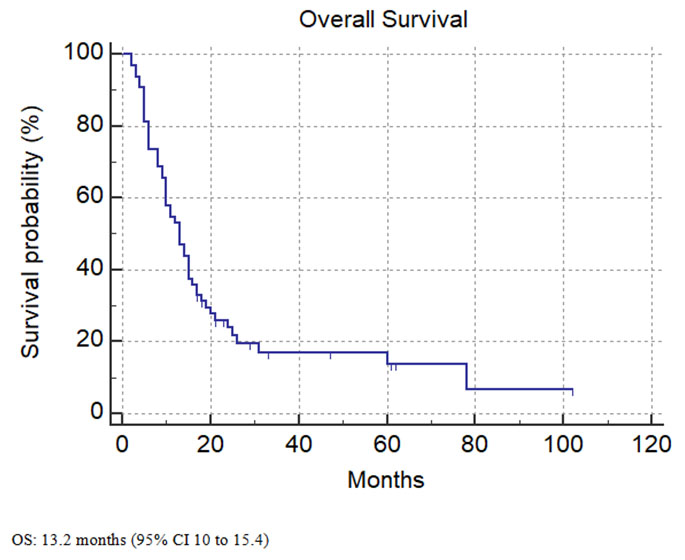
At the time of analysis, 82.9% of the patients had died. The median overall survival was 13.2 months (95% CI 10–15.4) (Figure 1).
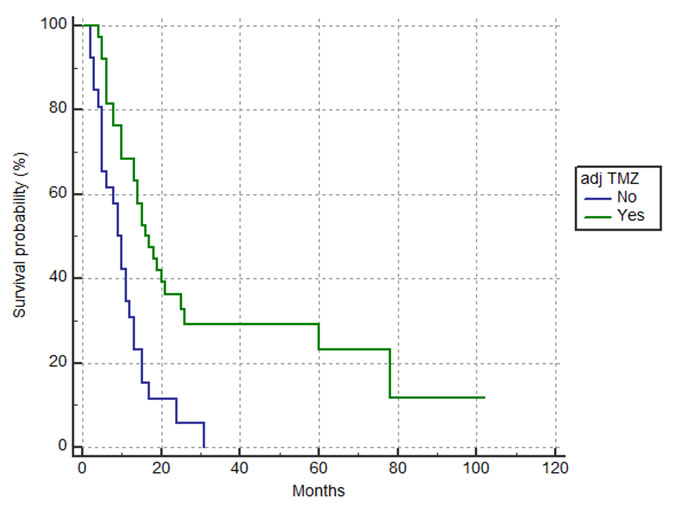
Patients who received adjuvant TMZ with more than one cycle had a significantly better survival outcome of 15.6 months (95%CI 13.2–25.7) compared to patients who did not receive adjuvant TMZ or received only one cycle 9.6 months (p=0.004) (Figure 2).
On the other hand, median OS for patients who had surgical resection was longer than patients who had biopsy only, 14.5 compared to 11.1 months (p=0.62).
Median OS was not reached for patients with methylated MGMT status compared to a median OS of 10.7 months in patients with an unmethylated MGMT status (p=0.37).
Progression of disease was the cause of treatment discontinuation in 42% of the patients, followed by toxicities in 17%. Thrombocytopenia was the most frequent toxicity leading to treatment discontinuation in about 7.8% of the population (Table 2).
At the time of analysis, 11 patients (17.1%) were alive, 5 under regular radiological surveillance, 2 had resection only for recurrence, 2 receiving the second line with bevacizumab, 1 had received further palliative RT followed by TMZ for recurrence, and 1 patient treated with palliative RT for recurrence and continued in surveillance.
DISCUSSION
The Stupp protocol has become part of the standard of care for managing patients with GBM [13]. We aim to review the outcomes of patients treated with Stupp protocol in our Cancer Center over ten years. Our study found a median OS of 13.2 months, lower than the 14.6 months median OS in the published Stupp trial [13]. Although this is a slightly inferior result, this may be related to the smaller sample size in our study and a higher percentage of older patients treated in our center, with almost 80% of patients being 50 years old or older, and about 50% of the patients were above 60. Another factor could be the delay of adjuvant RT/TMZ treatment initiation in our study, 7.7 weeks compared to five weeks in the Stupp trial. Although there are conflicting studies on the optimal timing of initial cranial radiation in the treatment of GBM, multiple studies demonstrated that significant delays longer than six weeks might negatively affect OS [15],[16].
The benefit of adjuvant TMZ after RT-TMZ was significantly demonstrated in the survival outcome; patients who received adjuvant TMZ with more than one cycle had a median OS of 15.6 months compared to 9.6 months for patients who did not proceed with adjuvant TMZ or had only one cycle of adjuvant TMZ (p=0.004). A small number of patients, four, received adjuvant TMZ beyond the standard of six cycles. Although this is not standard practice, some neuro-oncologists may offer more than six cycles of adjuvant treatment if well tolerated; however, the benefit of extended adjuvant TMZ in prolongation of OS is not confirmed [17],[18].
Long-term results of the Stupp trial [14] indicated that patients with methylated MGMT GBM are most likely to benefit from the addition of TMZ. Our analysis demonstrated a similar result; however, our sample is very small, and the result was not statistically significant p=0.37.
The extent of surgery remains a significant prognostic variable as published in the literature [7],[8],[9]. In our study, 3.4 months increase in OS was observed in patients who had a surgical resection compared to patients who had a biopsy only, although this was not statistically significant, p=0.62.
CONCLUSION
In conclusion, the survival outcomes for patients with GBM treated with the Stupp protocol in our center were slightly inferior to those of the published Stupp data. Inferior OS outcome may be explained by a higher percentage of the older population treated in our center and a longer time from surgery to initiation of adjuvant treatment.
REFERENCES
1.
Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 2013;15(Suppl 2):ii1–56. [CrossRef]
[Pubmed]

2.
Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev 2014;23(10):1985–96. [CrossRef]
[Pubmed]

3.
Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer 2005;104(12):2798–806. [CrossRef]
[Pubmed]

4.
Young RM, Jamshidi A, Davis G, Sherman JH. Current trends in the surgical management and treatment of adult glioblastoma. Ann Transl Med 2015;3(9):121. [CrossRef]
[Pubmed]

5.
Davis ME. Glioblastoma: Overview of disease and treatment. Clin J Oncol Nurs 2016;20(5 Suppl):S2–8. [CrossRef]
[Pubmed]

6.
Schiff D, Lee EQ, Nayak L, Norden AD, Reardon DA, Wen PY. Medical management of brain tumors and the sequelae of treatment. Neuro Oncol 2015;17(4):488–504. [CrossRef]
[Pubmed]

7.
Stark AM, van de Bergh J, Hedderich J, Mehdorn HM, Nabavi A. Glioblastoma: Clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg 2012;114(7):840–5. [CrossRef]
[Pubmed]

8.
Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol 2011;13(12):1339–48. [CrossRef]
[Pubmed]

9.
Roder C, Bisdas S, Ebner FH, et al. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: High-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol 2014;40(3):297–304. [CrossRef]
[Pubmed]

10.
Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol 1999;52(4):371–9. [CrossRef]
[Pubmed]

11.
Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg 2001;95(2):190–8. [CrossRef]
[Pubmed]

12.
13.
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352(10):987–96. [CrossRef]
[Pubmed]

14.
Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10(5):459–66. [CrossRef]
[Pubmed]

15.
Irwin C, Hunn M, Purdie G, Hamilton D. Delay in radiotherapy shortens survival in patients with high grade glioma. J Neurooncol 2007;85(3):339–43. [CrossRef]
[Pubmed]

16.
Sun MZ, Oh T, Ivan ME, et al. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J Neurosurg 2015;122(5):1144–50. [CrossRef]
[Pubmed]

17.
Blumenthal DT, Gorlia T, Gilbert MR, et al. Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: A secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol 2017;19(8):1119–26. [CrossRef]
[Pubmed]

18.
Skardelly M, Dangel E, Gohde J, et al. Prolonged temozolomide maintenance therapy in newly diagnosed glioblastoma. Oncologist 2017;22(5):570–5. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Ruba A Hamed - Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Thamir Mahgoub - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Laura Lowry - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mazin Elbassiouni - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Greg Korpanty - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Ruba A Hamed et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.